
Green Science Alliance Has Started Supplying Solid Electrolyte Li7La3Zr2O12 (LLZO) with Garnet Type Structure for Solid-State Lithium Ion Battery
KAWANISHI, Japan: Global warming is becoming a serious issue because of the increase in greenhouse gases such as methane and carbon dioxide (CO2). There has been an increase in the production of hybrid, electric, and fuel cell cars to reduce of CO2 emissions from automobiles. Highly functional, rechargeable batteries are ideal for this purpose. Lithium ion batteries have been widely used. For the smart-grid society, rechargeable batteries with high capacity are able to store electricity produced by sustainable energy sources such as wind power and solar cells. However, the battery capacity of currently used lithium ion batteries is approximately 100 – 240 Wh / kg. A battery that is safer to use should be created since lithium ion batteries are flammable due to the flammable electrolyte they contain. In addition, lithium ion battery with higher battery capacity is desired. Solid-state lithium ion batteries are ideal because they are robust and safe.
Furthermore, theoretical capacity of all solid-state lithium ion battery is higher than current one. Research and development are ongoing at universities, research institutes, and companies all over the world to develop solid-state lithium ion batteries. The most important technology for these batteries is the creation of a solid electrolyte in which lithium ion can move and transfer efficiently between the cathode and anode during electrochemical reactions. There are two main types of solid electrolytes: one is based on organic polymer, and the other is inorganic type. In view of safety and robustness, inorganic solid electrolyte is ideal although the research history of inorganic solid electrolyte is not as long as the organic electrolyte, and its safety and detailed reaction mechanism are still unknown.
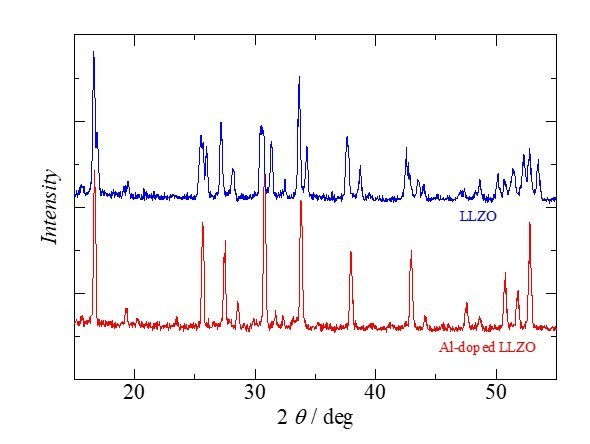
Because of their robustness, however, inorganic solid electrolytes are more favorable and aggressively being developed by many researchers. Solid electrolytes can be largely classified into sulfide-based and oxide-based materials. There are a number of research reports that a sulfide-based inorganic solid electrolyte battery can possess a capacity higher than that of an oxide-based battery although sulfide causes toxic hydrogen sulfide when exposed to ambient air. Therefore, the battery needs to be hermetically sealed; the manufacturing cost would not be economically friendly. Thus, Green Science Alliance has been focusing on oxide-based solid electrolytes because they are more stable and robust, and the manufacturing process is more scalable than that of sulfide. Oxide solid electrolytes mainly include NASICON-type Li1.3Al0.3Ti1.7(PO4)3(LATP), as perovskite-type La0.34Li0.51TiO3, and garnet-type Li7La3Zr2O12(LLZO). LLZO exhibits high conductivity (from 10−4 to 10−3 S cm−1) even at room temperature. In addition, LLZO is the only oxide-based solid electrolyte that is stable toward Li metal.
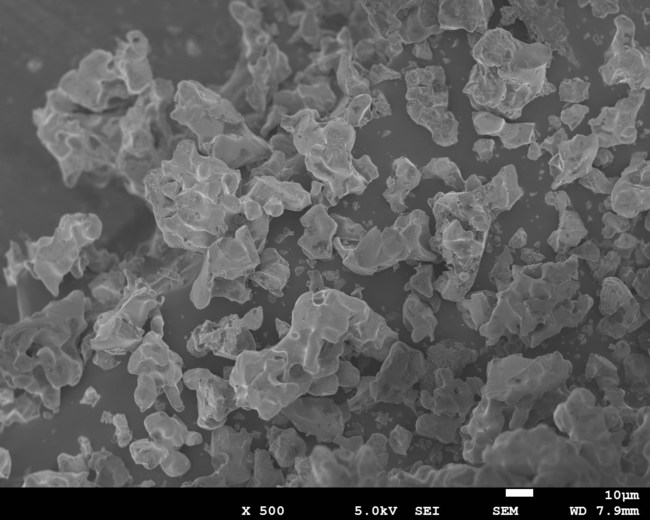
Furthermore, Green Science Alliance Co., Ltd. has been successfully synthesizing Al-doped LLZO, which possesses even higher conductivity. Green Science Alliance also synthesizes various types of electrodes and can prepare a small testing-scale lithium ion battery internally in the company for their electrochemical measurements. Various types of electrodes and ionic-liquid-based electrolytes can be synthesized on their own and combined with currently developed LLZO. They will challenge to create the next generation of lithium ion batteries, including all solid-state lithium ion batteries. The company also analyzes materials and prepare the battery following standard procedures such as electrochemical measurements, X-ray diffraction patterns, and electron microscopy. They will start supplying a sample of LLZO powder and inks. The size of the LLZO powder particles is larger than several micrometers at synthesis although they can be as small as 200 – 300 nm with nanosize dispersion technology with after process. The next challenge is to prepare LLZO of smaller particle size.
















